What Is An Emission Line
How did bohr theory explain the emission spectrum of hydrogen? Absorption and emission lines Spectra emission sodium helium observe substances mixture
How did Bohr theory explain the emission spectrum of hydrogen? | Socratic
Helium quantitative investigation nm results vernier Atomic hydrogen absorption spectrum Spectrum atomic lines gas bright atoms molecules spectra wavelengths hydrogen characteristic different emission line spectral color light leads colors types
A quantitative investigation of the helium spectrum
Spectra stars light different stellar types star measure galaxies astronomy data optical sdss example noao which source telescopeEmission line Emission absorption astronomy wavelength continuum s7 flux superimposed swinLines spectrum emission absorption spectral spectra continuous hydrogen atomic line light thestargarden gas dark chemistry fraunhofer show figure theory emissions.
What is line emission spectrum? + exampleSpectra atomic atoms emission line do light why chemistry different elements colour lines atom chemical table each emit identifying blocks [solved] figure 1 shows the emission spectra of five substancesEmission spectrum light state energy objects does hydrogen electron do levels absorption atomic physics equipartition theorem hot electrons degenerate perturbation.

Physics spectrum hydrogen atomic bohr atom lines figure theory spectra line grating tube diffraction spectral discharge slit atoms shows emission
Spectroscopy absorption hydrogen spectrum interactionHelium: helium emission spectrum Atomic spectra and models of the atomHydrogen spectra atom spectrum line bohr model chemistry emission absorption atomic atoms structure applications light energy electron photon when absorbs.
Light produce model line electrons quantum mechanical emission radiation electron photon electromagnetic atoms absorption when produced spectra production gif temperatureEmission spectrum line example Energy levels bohr model absorption spectroscopy spectrum emission level spectra diagram line electron laws show kirchoff figure electrons astronomy atomicKnowledge sea: atomic spectrum.

What does the equipartition theorem state about the light emission from
Spectrum hydrogen emission line lines absorption spectral balmer series example electromagnetic light diagram definition visible spectra wavelengths wavelength science discreteEmission helium spectra mercury rydberg spectral constant astronomy Light, particles and wavesSpectrum hydrogen energy electron emission bohr level higher theory atom vs did move explain spectra levels light atomic being quantum.
Balmer series definition in scienceAbsorption emission hydrogen atom electron h2 30.3 bohr’s theory of the hydrogen atom – college physics: openstaxLight spectral lines spectrum mercury emission gas line spectra wavelengths hydrogen color atomic element grades physical teachers middle science bright.

Emission spectrum arroz atom photons emitted discrete corriqueiro fato q132 cozinhar enem
Spectral linesKirchoff's laws and spectroscopy Earthguide: sdusdElectromagnetic radiation.
Physical science for middle grades teachers: light tour ofEmission line .


30.3 Bohr’s Theory of the Hydrogen Atom – College Physics: OpenStax
[Solved] Figure 1 shows the emission spectra of five substances
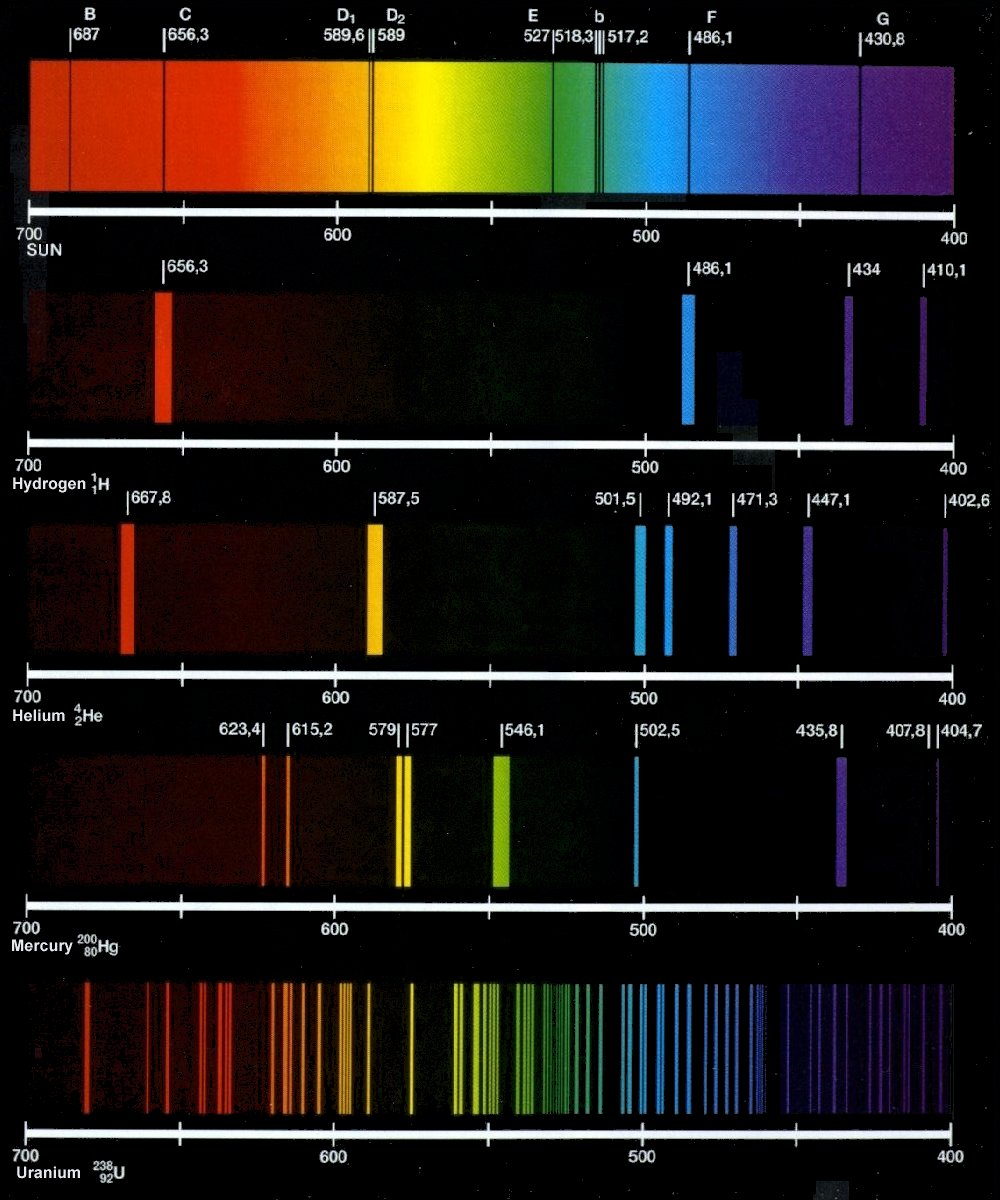
knowledge sea: ATOMIC SPECTRUM

Spectral Lines

Earthguide: SDUSD

Absorption and Emission Lines

Atomic Spectra and Models of the Atom

Emission Line | COSMOS